Questions about climate science
Some people may want to understand more than is given on this website, either out of general interest, or because they have seen material from “climate deniers” – people who claim that the generally accepted science about global warming is either downright wrong, unsubstantiated or that climate change is less dangerous than generally supposed.
As humanity across the world will have to change our ways of living very substantially, it is as well to be convinced that climate science is indeed correct.
The world’s scientists declare a climate emergency
An open letter was signed by scientists from around the world was published on 5 November 2019 in the journal BioScience). The authors have tracked the Earth’s ‘vital signs’ over the last 40 years. These include rainforest loss, carbon emissions, human energy consumption, ocean acidity and sea level rise, which are all linked to climate change. The letter includes 11,258 signatures from 153 countries. “We declare … clearly and unequivocally that planet Earth is facing a climate emergency,” says the letter’s opening statement. All the details of those who signed the endorsement have been published online.
Skeptical Science Website
If you have specific questions you may well find responses on the Skeptical Science website. There is a list of nearly 200 “Climate Myths” and each one has up to three scientific explanations pitched at different levels.
For example, some people doubt that carbon dioxide could have such an effect. See Questions 30 and 77 for full scientific explanations.
What is “The Greenhouse effect”?
Very small amounts of carbon dioxide in the atmosphere can change the proportion of the sun’s heat that is absorbed and this can disrupt weather patterns quite severely.
The glass of a greenhouse acts in a similar way which is why greenhouses get so warm.
There are other gases that have a similar effect, notably methane, but for convenience scientists often use “carbon dioxide equivalents” to describe what is happening.
There a lot of more in-depth explanations online: try the Skeptical Science website question 30.
Gases in the atmosphere
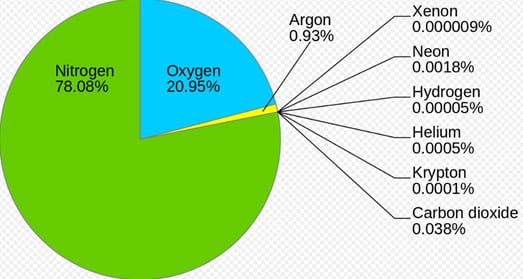
Carbon dioxide facts
Carbon dioxide is present in tiny amounts. This diagram shows 0.038% but more recent figures put it at 0.041%. This equates to 41 parts per million.
Carbon dioxide being taken in by plants and breathed out by animals has always been naturally balanced. (Until the industrial age started to use vast amounts of fossil fuels from millions of years ago and overwhelmed that balance).
Extra carbon dioxide pumped artificially into greenhouses improves crop yields, so is good for plants in that sense.
Changes in carbon dioxide concentrations have a huge effect on global temperature in spite of the seemingly tiny amount present.
Tiny increases in the amount of carbon dioxide in the atmosphere cause a build up of trapped heat and a destabilising of climate systems.
Oxygen is required for all life except for a few types of “anaerobic” bacteria.
Nitrogen makes up the bulk of our atmosphere and is largely inert in its atmospheric form. Nitrogen compounds are present in the soil and are required for plant growth. (Remember the “bulk” of the plant is made up of carbon compounds formed during photosynthesis).
Argon is an “Inert gas” and does not react with anything. It was previously used inside light bulbs to help preserve the electrical element.
See Skeptical Science 77 – CO2 is just a trace gas.
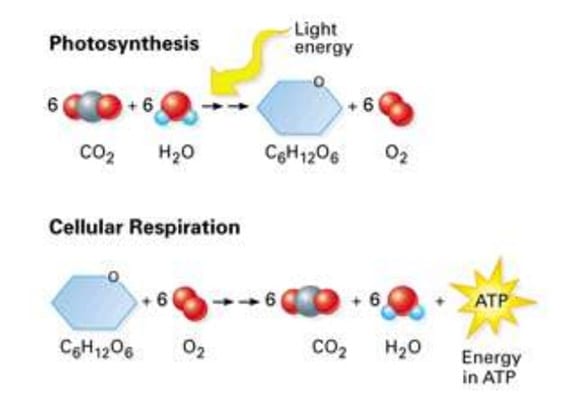
What is the Carbon Cycle?

Green plants take in carbon dioxide to create sugars that are processed to build the bulk of the plant’s structure.
(This process is called photosynthesis. It uses sunlight as its energy source to combine carbon dioxide and water to make sugars in green leaves. This process also releases oxygen.)
All proteins, fats and carbs are made from these sugars.
Animals (including humans) eat plants (or other animals that have in turn eaten plants) and so they get all their building blocks for growth and energy from photosynthesis.
Animals use oxygen to process the sugars they have eaten to produce energy for life. Carbon dioxide and water are given off.
This is called respiration. It is the reverse of photosynthesis.
(Plants also get their energy from respiration, but the intake of oxygen and output of carbon dioxide is very small compared to the reverse process of photosynthesis)
This completes the carbon cycle and for millennia the amounts of oxygen and carbon dioxide going in and out of the atmosphere have been in balance.
So what happened to unbalance it?
- Coal, oil and natural gas (fossil fuels) were formed from plants and sea creatures millions of years ago over vast stretches of time.
- Humans have dug up and burned millions of year’s worth of fossil fuels in just a few decades releasing all that carbon dioxide effectively “all at once.”
No wonder the balance has been thrown off and the atmosphere and climate systems are changing so fast.
Planting millions of trees will help the climate re-stabilise, but that on its own won’t be enough.
Atoms and Molecules
ATOMS
You will remember that atoms are the particles that make up all chemical matter. They have a number of protons and neutrons making up the nucleus and also a number of electrons forming a sort of cloud around the nucleus. Typically the number of protons, neutrons and electrons in an atom are the same. For example, an atom of Helium comprises of 2 protons, 2 neutrons and 2 electrons. Atoms and molecules are incredibly small, so you cannot really say what they “look like”. These diagrams just give an idea of their shapes.
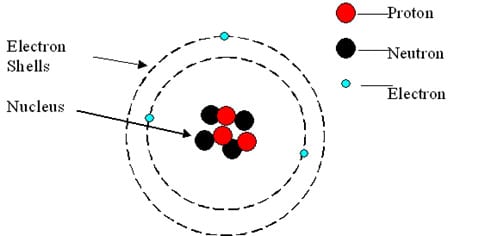
Extra info: This atom has 3 protons so it is Lithium. It also has 3 electrons (one of which is easily lost in chemical reactions). Lithium exists in two different forms, one with 3 and the other with 4 neutrons.
MOLECULES
Many substances exist as molecules where 2 or more atoms are grouped together. If the molecule contains all the same type of atom we have an element. For example atmospheric consists of molecules of oxygen. Each molecule has two atoms of oxygen. In molecular diagrams, oxygen is usually shown as red. Molecules of oxygen can form with 3 atoms and then it is called ozone.
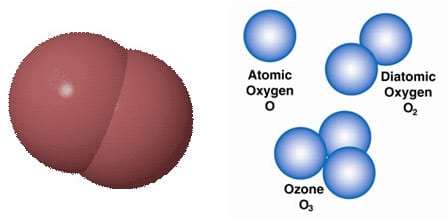
Ozone is present in very low concentrations throughout the atmosphere, with its highest concentration high in the ozone layer of the stratosphere, which absorbs most of the Sun’s ultraviolet (UV) radiation. Damage to the ozone layer can cause skin damage in humans. An “ozone hole” was first discovered in 1982. The main cause was manufactured chemicals, especially CFC refrigerants. The hole is now largely repaired as CFCs have been banned.
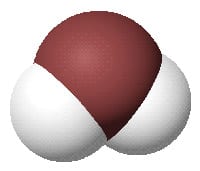
Water molecules
The formula for water is H2O. That means each molecule of water is made of one oxygen atom and two hydrogen atoms. Hydrogen atoms are usually shown as white.
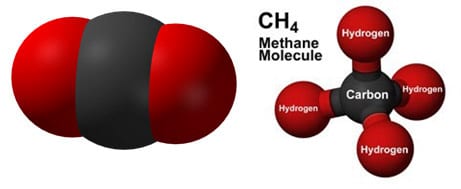
Carbon dioxide molecule and methane molecule
Carbon atoms are generally shown as black in diagrams. Carbon can make a vast number of different compounds, simple ones such as the ones shown below and complex ones such as hydrocarbons, carbohydrates, proteins and all the molecules of life. Hydrocarbons (hydrogen and carbon with no oxygen) are found in fossil fuels which of course were originally formed from trees and sea life.
Carbon monoxide and sugar (glucose)
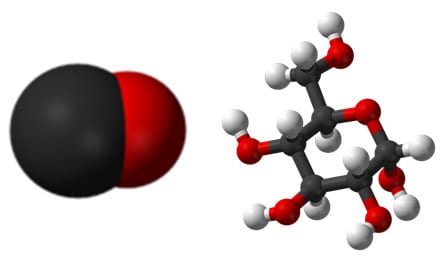
Note: The type of diagram is often a bit different for larger molecules for practical reasons.
Molecular diagrams
Methane
When released into the atmosphere methane causes much more warming than carbon dioxide, but is broken down much more quickly. When it does break down it forms carbon dioxide and water exactly as is if it had been burned.
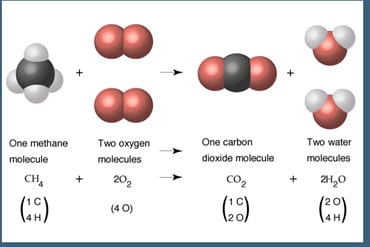
So it is much better to burn methane, the carbon dioxide will last in the atmosphere anyway and it’s better not to have the couple of decades of the extra warming that the methane gives.
When the methane comes from biomass then the carbon is part of the ‘natural’ carbon cycle rather than from a fossil fuel (such as natural gas).
- Biofuel is better than natural gas (they are both methane)
- Natural gas is better than other fossil fuels such as coal and oil.
Renewables such as solar or wind are, of course, better still.
 Alton Climate Action Network
Alton Climate Action Network